Shares of vaccine-maker Moderna MRNA closed at $34.71 on Monday, close to their 52-week low of $29.25.
Moderna is facing several headwinds, including the declining COVID-19 vaccine and the soft sales performance of its recently launched RSV vaccine. There have also been several third-party reports that the U.S. government is re-evaluating the $590 million contract that was recently awarded to the company for the development of an mRNA-based vaccine for bird flu.
In the past year, MRNA stock has lost two-thirds of its market value, significantly underperforming the industry, as seen in the chart below. During this timeframe, the stock also underperformed the broader Medical sector and the S&P 500. Shares of Moderna are currently trading below their 200-day and 50-day moving averages.
MRNA Stock Underperforms Industry, Sector & S&P 500
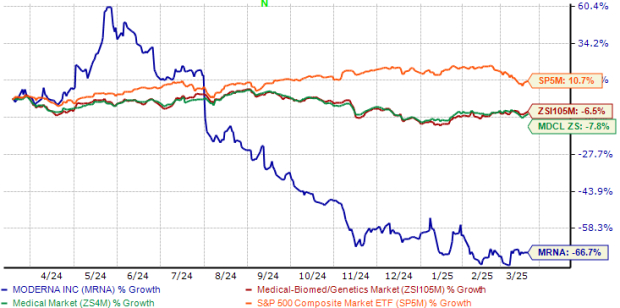
Image Source: Zacks Investment Research
The stock’s poor price performance has left investors wondering whether to buy, hold or sell it. Let’s understand the company’s strengths and weaknesses in detail to better analyze how to play the stock following the price decline.
Competition From Pharma Giants: A Hurdle for Moderna
While Moderna's mRNA technology gives it a competitive edge, its products face competition from several large pharmaceutical players like GSK plc GSK and Pfizer PFE.
Last year, Moderna secured its first product approval outside the COVID-19 vaccine space when the FDA approved the company’s RSV vaccine, mResvia. Though many stakeholders had initially perceived this approval as a turning point for Moderna to diversify its revenue stream beyond COVID-19 vaccines, the underwhelming sales performance of this vaccine disappointed investors. The Moderna vaccine faces stiff competition from RSV vaccines Arexvy and Abrysvo, marketed by GSK and Pfizer, respectively.
Moderna’s Encouraging Upcoming Product Launches
Moderna expects to launch 10 new products over the next three years and has made considerable progress toward this goal. Toward the end of last year, the company submitted three regulatory filings to the FDA — one for mRNA-1283 (next-generation COVID-19 vaccine) and another for mRNA-1083 (COVID-19 and influenza combination vaccine). A final decision from the FDA on mRNA-1283 is expected by May 31.
The third regulatory filing seeks expanded use of the mResvia in high-risk adults aged 18-59. A final decision on the mResvia filing is expected by June 12. Currently, mResvia is approved for use in older adults aged 60 and above.
With these product launches, Moderna aims to boost its revenues and reduce its dependence on the COVID-19 vaccine, which remains a major contributor despite the falling sales.
MRNA’s Pipeline Progress Holds Potential
The company is also progressing well with the development of its pipeline candidates. Unlike traditional vaccines that can take months to produce, mRNA-based vaccines can be developed quickly and offer manufacturing scalability. This major advantage has been a plus point for Moderna and Pfizer, which have been able to update their mRNA-based COVID-19 vaccines against the latest variants at record time, especially when compared to Novavax.
Moderna has more than 40 mRNA-based investigational candidates in different stages of clinical studies, targeting various indications, including cancer. It is evaluating multiple candidates in late-stage studies, including mRNA-4157/V940 (individualized neoantigen therapy), mRNA-1647 (cytomegalovirus [CMV] vaccine) and mRNA-1010 (influenza vaccine).
mRNA-4157 is an important candidate garnering investors’ attention. It is being developed in partnership with Merck MRK. Moderna/Merck is evaluating this therapy in three pivotal late-stage studies — one in the melanoma indication and the other two in the non-small cell lung cancer area.
Moderna/Merck is also evaluating mRNA-4157 across separate mid-stage studies for cutaneous squamous cell carcinoma, renal cell carcinoma and muscle-invasive bladder cancer indications.
Data readout from the late-stage study on the CMV vaccine is expected later this year.
MRNA Stock Valuation & Estimates
From a valuation standpoint, Moderna appears to be trading at a premium compared to the industry. Going by the price/sales (P/S) ratio, the company’s shares currently trade at 4.13, trailing 12-month sales value, higher than 2.20 for the industry.
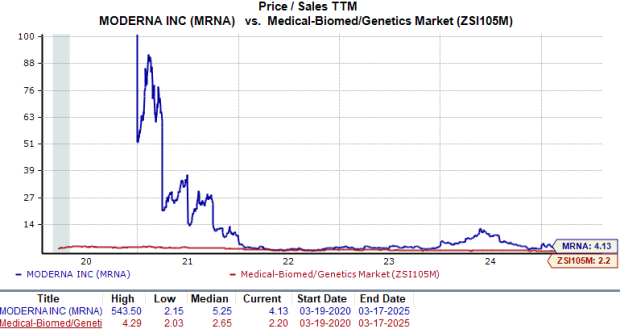
Image Source: Zacks Investment Research
Estimates for Moderna’s 2025 loss per share have widened from $8.98 to $10.03 in the past 60 days.During the same period, estimates for 2026 loss per share have widened from $6.65 to $7.02.
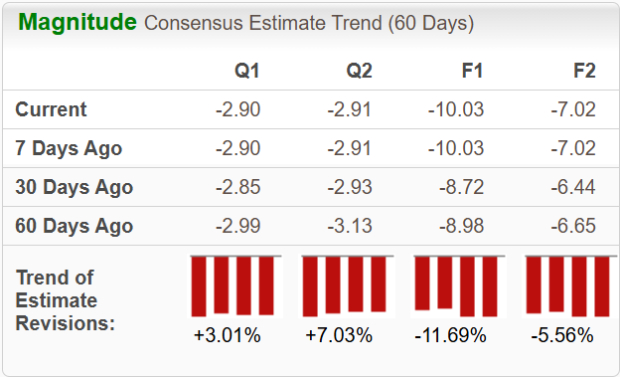
Image Source: Zacks Investment Research
How to Play MRNA Stock?
We advise investors with a long-term horizon to stay invested in this Zacks Rank #3 (Hold) company based on the strong cash balance ($9.5 billion as of 2024-end) and robust pipeline progress. The quick development of mRNA-based vaccines gives Moderna an edge over its rivals, especially those developing traditional vaccines. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
However, short-term investors are requested to exercise caution when investing in the stock mainly due to concerns over top-line growth. The consistently declining earnings estimates highlight analysts’ pessimistic outlook for the stock. Moderna shares are also currently trading at a premium to the industry.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2024. While not all picks can be winners, previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GSK PLC Sponsored ADR (GSK): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Moderna, Inc. (MRNA): Free Stock Analysis Report
This article originally published on Zacks Investment Research (zacks.com).


/AI%20(artificial%20intelligence)/3D%20Graphics%20Concept%20Big%20Data%20Center%20by%20Gorodenkoff%20via%20Shutterstock.jpg)
/Netflix%20on%20tv%20with%20remote%20by%20freestocks%20via%20Unsplash.jpg)

/Nvidia%20logo%20and%20sign%20on%20headquarters%20by%20Michael%20Vi%20via%20Shutterstock.jpg)
